Potassium-magnesium salts are composed of the following key minerals: sylvine, carnallite (potassium and magnesium chlorides), polyhalite, langbeinite and kieserite (potassium and magnesium sulphates) and kainite (a mixed mineral).

Rock salt with carnallite (hydrated potassium-magnesium chloride – KMgCl3· 6H2O)
from Soligorsk, Belarus. Photo by: Leonid Gulis
These salts share many characteristics with common rock salt (halite – sodium chloride) and occur alongside it. They are translucent, of a low hardness (can be scratched with fingernail), easily soluble in water (although sulphates much less so than the chlorides), deliquescent (i.e. are capable of absorbing moisture from the surrounding air). Unlike halite, however, they are all bitter in taste.

Sylvite (potassium chloride – KCl) From a salt dome in Kłodawa
Photo by: Jacek Wachowiak
In pure form, potassium-magnesium salts are white, but coloured (orange, blue, green, etc.) varieties commonly occur in the environment. This is due to the inclusions of other minerals that contain compounds of iron, copper or of other elements.

Polyhalite from the Puck bay (hydrated sulphate of potassium-magnesium- -calcium – K2MgCa2[SO4]4· 2H2O)
Photo by: Katarzyna Skurczyńska-Garwolińska
Those unpalatable table salt relatives started their international career as late as in the middle 19th century on discovery of benefits from boosting plant growth by adding mineral compounds to the soil. Before long, mass production of artificial fertilizers followed the discovery. Today, the potassium-magnesium salts are indispensable for the agriculture. They are behind the several-fold increase in crop yield over the past two centuries.
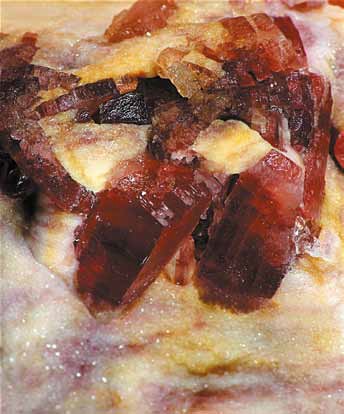
Crystals of anhydrite (claret) within polyhalite from the Mogilno salt dome. Polyhalite is a hydrated sulfate of potassium-magnesium- -calcium (K2MgCa2[SO4]4· 2H2O)
Photo by: Jacek Wachowiak








