Like rock salt deposits, potassium-magnesium salts were deposited as a result of water evaporation from shallow seas, lagoons or lakes in hot and dry climate conditions. Generally, this process occurs in abandoned water reservoirs, with a limited supply of seawater (lagoons and seashore ponds case) or fresh water recharged by rivers (continental saline lakes). Water salinity in the reservoir increases with progressive evaporation so that mineral compounds start to precipitate on the banks and the bottom of the reservoir.
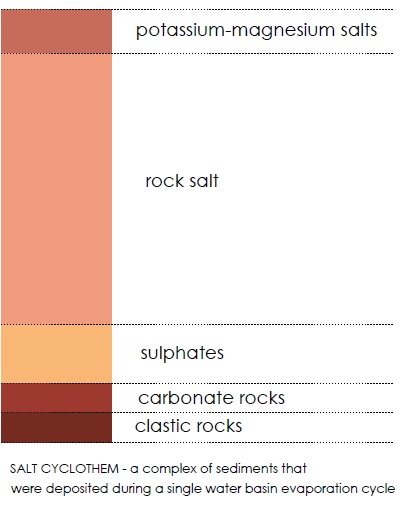
Minerals always precipitate from brine water in a strictly defined order that is controlled by salt concentration in the solution. Calcium and magnesium carbonates (calcite, aragonite and dolomite) are the first to precipitate and form carbonate rocks. These rocks are also formed on the bottom of the seas and oceans with normal salt concentrations, but the process is slightly slower there. As the brine water is getting more condensed, gypsum and anhydrite (calcium sulphates) begin to precipitate, followed by rock salt (halite – sodium chloride) and, ultimately, by potassium-magnesium salts.
Thick salt seams, that are today buried under other sediments deep in the ground, reveal that in the history of our planet several times a huge salt water body of Mediterranean Sea size or larger has evaporated over a short period of time.
Today, the process of salt precipitation can be observed on the fast shrinking Dead Sea or several salt desert lakes, e.g. Great Salt Lake, located in the northern part of the U.S. state of Utah.

Salt mushroom on the shore of the Dead Sea. Photo by: Grzegorz Czapowski
Comparing to rock salt, potassium-magnesium salts occur less frequently and in smaller quantities, although the abundance of sodium and potassium in Earth’s crust is similar. There are several reasons behind that pattern: first – potassium derived from rock weathering is retained in the soil and taken up by plants; second – potassium-magnesium salts are the final product of water basin evaporation, while a complete evaporation of the entire sea was not a common phenomenon in Earth’s history; and third – potassium magnesium salts, being the most soluble of all deposited salts, are quickly reduced by erosion.
Did you know that...
A 1 to 10 cm thick layer of salt is precipitated over a period of one year.
Salt pan Salar de Atacama, Chile. Photo by: Krzysztof Bukowski








